Multiple Sclerosis
The prevalence of multiple sclerosis (MS) has been rising since 2013, and as of 2020 it was estimated to affect 2.8 million individuals globally 1. Significant progress has been made in recent decades in the understanding of MS, particularly the relapsing-remitting phase of the disease, and a number of effective disease modifying therapies (DMTs) are now available. However, many of these DMTs are associated with undesirable side effects and are ineffective in treating progressive forms of MS. Thus, there is an urgent need to develop novel therapies that are efficacious in progressive MS and exhibit an improved side effect profile.
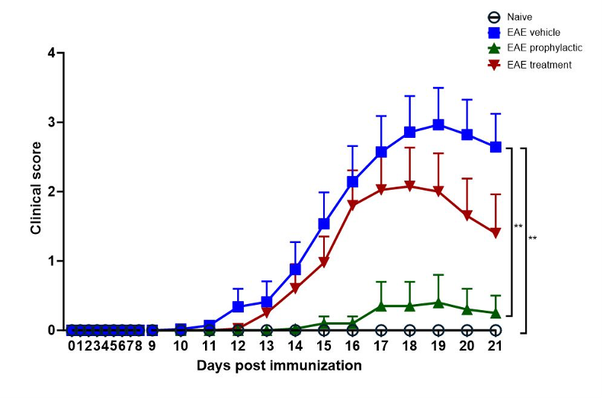
Experimental autoimmune encephalomyelitis (EAE) is a representative animal model, which manifests, like MS, CNS-related histopathological findings, motor deficits and, inflammatory responses. EAE is one of the most successful disease models in the CNS field, and now in its 90th year 2, the neuroimmune mechanisms responsible for the phenotype have been well described 3. The immunomodulator, fingolimod, a sphingosine 1-phosphate (S1P) modulator, is the first oral drug that has been approved for treating MS and has been broadly used as a positive control in the EAE mouse model 4-7.
Fingolimod treatment prevents disease progression in EAE
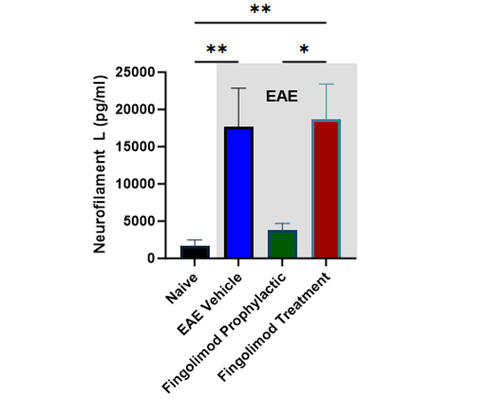
Translational biomarkers: Increased plasma neurofilament light chain (NfL) in EAE is inhibited by fingolimod
The mechanisms of disease manifestation in EAE are well established. Therefore, the model can be broadly used for neuroimmunology programs beyond MS. Clinical score and NfL measurements can be combined with other analyses to provide a more comprehensive profiling of compound efficacy:
- Quantification of cytokine levels in CNS, spleen, and plasma
- Western immunoblotting to evaluate selected signalling pathways (e.g. NLRP3)
- CNS histopathology
- Walton C, King R, Rechtman L, Kaye W, Leray E, Marrie RA, Robertson N, La Rocca N, Uitdehaag B, van der Mei I, Wallin M, Helme A, Angood Napier C, Rijke N, Baneke P. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult Scler. 2020 Dec;26(14):1816-1821. doi: 10.1177/1352458520970841. Epub 2020 Nov 11. PMID: 33174475; PMCID: PMC7720355.
- Steinman L, Patarca R, Haseltine W. Experimental encephalomyelitis at age 90, still relevant and elucidating how viruses trigger disease. J Exp Med. 2023 Feb 6;220(2):e20221322. doi: 10.1084/jem.20221322. Epub 2023 Jan 18. PMID: 36652203; PMCID: PMC9880878.
- Constantinescu CS, Farooqi N, O'Brien K, Gran B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br J Pharmacol. 2011 Oct;164(4):1079-106. doi: 10.1111/j.1476-5381.2011.01302.x. PMID: 21371012; PMCID: PMC3229753.
- Barthelmes J, Tafferner N, Kurz J, de Bruin N, Parnham M J, Geisslinger G, Schiffmann S. Induction of Experimental Autoimmune Encephalomyelitis in Mice and Evaluation of the Disease-dependent Distribution of Immune Cells in Various Tissues. J. Vis. Exp. (111), 2016 May, e53933, doi:10.3791/53933. PMID: 2721439; PMCID: PMC4942059.
- Bonfiglio T, Olivero G, Merega E, Di Prisco S, Padolecchia C , Grilli M, Milanese M, Di Cesare Mannelli L, Ghelardini C, Bonanno G, Marchi M, Pittaluga A. Prophylactic versus Therapeutic Fingolimod: Restoration of Presynaptic Defects in Mice Suffering from Experimental Autoimmune Encephalomyelitis. PLoS ONE 12(1), 2017 January, e0170825, doi:10.1371/journal. PMID: 28125677, PMCID: PMC5268435.
- Derfuss T, Mehling M, Papadopoulou A, Bar-Or A, Cohen J A, and Kappos L. Advances in oral immunomodulating therapies in relapsing multiple sclerosis. Lancet Neurol 19, 336–347, 2020 Feb, doi: 10.1016/S1474-4422(19)30391-6. PMID: 32059809.
- Yang T, Zha Z, Yang X, Kang Y, Wang X, Tong Y, Zhao X, Wang L and Fan Y. Neuroprotective Effects of Fingolimod Supplement on the Retina and Optic Nerve in the Mouse Model of Experimental Autoimmune Encephalomyelitis. Front. Neurosci. 15:663541, 2021 April, doi: 10.3389/fnins.2021.663541.